Manual 04 for MD++
Creating a Vacancy, Relaxing The Imperfect Structure
and Calculating Vacancy Formation Energy
Keonwook Kang and Wei Cai
Contents |
Making a Vacancy
In manual M02, we learned how to create a perfect crystal using MD++. However, most crystal structures are hardly perfect in natural environmet. It is common to have various kinds of defects such as point, line, planar and volume defects. Sometimes, we intend to introduce certain kinds of defects to study the property of interest.
Vacancy is one of the frequently encountered point defects in solids and can be considered as a missing atom from the otherwise perfect crystal. In this manual, we will learn how to create a vacancy inside of the perfect crystal and how to calculate the vacancy formation energy of the bulk material. Let’s run the following example script by typing
$ bin/fs gpp scripts/movacancy.script
# -*-shell-script-*-
setnolog
setoverwrite
dirname = runs/movacancy # specify run directory
#--------------------------------------------
# Read in potential file
potfile = ~/Codes/MD++/potentials/mo_pot readpot
#--------------------------------------------
#Create Perfect Lattice Configuration
crystalstructure = body-centered-cubic latticeconst = 3.1472 #(A)
latticesize = [ 1 0 0 5
0 1 0 5
0 0 1 5]
makecrystal finalcnfile = perf.cn writecn
eval # evaluate the potential of perfect crystal
#--------------------------------------------
# Create Vacancy
input = [ 1 # number of atoms to be fixed
0] # index of an atom to be fixed
fixatoms_by_ID # fix a set of atoms by their indices
removefixedatoms # remove fixed atoms
finalcnfile = movac.cn writecn
eval # evaluate the vacancy-formed crystal
#---------------------------------------------
# Plot Configuration
atomradius = 1.0 bondradius = 0.3 bondlength = 0
atomcolor = blue highlightcolor = purple backgroundcolor = gray
bondcolor = red fixatomcolor = yellow
plotfreq = 10 win_width = 600 win_height = 600
plot_atom_info = 3
color00 = "orange" color01 = "purple" color02 = "green"
color03 = "magenta" color04 = "cyan" color05 = "purple"
color06 = "gray80" color07 = "white"
plot_color_windows = [ 2 # number of color windows
-10 -6.8 6 # color06 = gray80
-6.7 -6.0 0 # color00 = orange
]
rotateangles = [ 0 0 0 1 ]
openwin alloccolors rotate saverot plot
sleep quit
The script above shows how to create a vacancy from the perfect BCC Mo crystal. First, a 5×5×5 perfect cubic crystal of Mo is created with all the simulation box edges aligned along <100> directions. Then an atom of ID 0 is fixed by
input = [ 1 # number of atoms to be fixed
0 ] # index of an atom to be fixed
fixatoms by ID
and will be removed by the command removefixedatoms in the script. Suppose, if you want to remove two atoms of indices 3 and 8, you can do so by
input = [ 2 # number of atoms to be fixed
3 8 ] # index of atoms to be fixed
fixatoms_by_ID # fix a set of atoms by their index
removefixedatoms # remove fixed atoms
At this moment, you may wonder how we know which atom has which index. If you click an atom in the visualization window with the left button of the mouse, it gives the index number and energy of the clicked atom.<ref> This is the case when plot_atom_info = 3 is specified in the script file. If plot_atom_info = 1, the index number and the reduced coordinate of the clicked atom will be given. If plot_atom_info = 2, the index number of the clicked atom and its real coordinate (in Å) will be displayed.</ref> In the visualization window, you will see the atoms near the vacant site have different color than the others. In the script file, two color windows are set by the variable plot_color_windows according to the potential energy of the individual atom. The atoms whose energies are between -10 and -6.8 (eV) are shown in gray and the atoms whose energies lie between -6.7 and -6.0 (eV) are shown in orange. It shows that the atoms near the vacancy have higher energy than the rest. Click the atoms and you will see the potential energy of each atom. If you also compare the potential energy of the perfect structure and the vacancy-formed structure, you will know which structure has higher energy.
Q.1 If a single atom, whose index is other than 0, is removed from the same perfect structure, do you still expect the final state has the same amount of energy? Explain the reason.
Relaxation
After creating a vacancy, it is not hard to imagine that the atoms around the vacancy will not stay as they were, for they have less bonds and form a locally unstable structure. We can expect the atoms near the vacancy will move toward the vacant site to some degree and lower the total energy of the system. In other words, the atomistic structure will be relaxed to its locay energy minimum state from the given initial position. MD++ uses a conjugate gradient relaxation (CGR) algorithm<ref> Numerical Recipes, http://www.nr.com/</ref> for this energy minimization process. for which the script file needs the section given below.
#--------------------------------------------- # Conjugate-Gradient relaxation conj_ftol = 1e-7 # tolerance on the residual gradient conj_fevalmax = 1000 # max. number of iterations conj_fixbox = 1 # fix the simulation box relax # CG relaxation command finalcnfile = relaxed.cn writecn eval # evaluate relaxed structure
Let’s copy the above script and paste it to the movacancy.script right before the last command, sleep quit. You will watch the potential energy of the simulated system keeps decreasing while the relaxation goes on. There are a coulple of variables related with CG relaxation. conj_ftol is the tolerance of the residual gradient and conj_fevalmax is the maximum number of iterations. The relaxtion will stop if the tolerance becomes smaller than the specified conj_ftol or if the number of iteration reaches its maximum. If conj_fixbox is equal to 1, the shape and size of simulation cell box is fixed so that only the atoms can move during the relaxation. Sometimes you need to relax the simulation box together with atoms. In such a case you can add more degrees of freedom to the system by setting conj_fixbox = 0. For example, you can allow the box to expand or shrink hydrostatically by saying that
conj_fixbox = 0
conj_fixboxvec = [ 0 1 1
1 0 1
1 1 0]
In this case, only the diagonal components of the box matrix 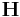 can relax and
the other components are fixed as assigned to conj_fixboxvec.
can relax and
the other components are fixed as assigned to conj_fixboxvec.
Vacancy Formation Energy
We define the vacancy formation energy Ev as the energy needed to create a vacancy from the bulk perfect crystal structure. This can be obtained by comparing the energy of the relaxed vacancy-formed structure and the energy of a perfect crystal structure. However, it is not just a simple substraction between two, because we need to make sure we compare two systems of the same number of atoms. In reality, no atom is removed or destroyed when a vacancy forms. Rather, what we can think of is displacement of an inside atom to the free surface of a solid leaving a vacancy inside as shown in Fig. 2(a). Even though there is no free surface in our simulation with the periodic boundary condition. Let N be the total number of atoms in the original perfect crystal structure. And E1 is the energy of the perfect crystal and E2 is the energy of relaxed structure of (N1) atoms. Then the vacancy formation energy Ev can be expressed as
N −1
Ev ≡ E2 − · E1 .
N
Q.2 Can you tell the different physical meanings between two expres
sions 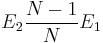 and E2E1? You can use Fig. 2 for your explanation.
and E2E1? You can use Fig. 2 for your explanation.
Once you know the vacancy formation energy Ev, you can use it to estimate the concentration of vacancy <maht>[n]</math> in the crystal strucutre at given temperature T and atmospheric pressure from Boltzmans distribution relation<ref> R. J. Stokes and D. F. Evans, Fundamentals of Interfacial Engineering, Wiley-VCH 1997 pp.491-493 </ref>,
[n] ∼ exp(−Ev /kB T )
where kB = 8.621E-5 eV/K is Boltzman’s constant. For example, the vacancy formation energy of Mo is 2.550 (eV)<ref> With the tight binding model, it is reported to be 2.46 eV. See the referene, M. J. Mehl and D. A. Papaconstantopoulos, “Applications of a tight-binding total-energy method for transition and noble metals: Elastic constants, vacancies, and surfaces of monatomic metals”, Physical Review B 54 4519-4530 1996 </ref> from the atomistic calculation. Then the fraction of vacancy will be on the order of 1.514E-43 at 300 K.
Q.3 Calculate the vacancy formation energy of Mo with the simulation box of different size. The vacancy formation energy is expected to be the same irrelavant to the box size. However, you will find the energy dramatically changes if the box is smaller than the certain size. Explain the reason.